what ratio is used to carry out each conversion
12.2: Mole Ratios
- Page ID
- 53790
Suppose that yous want to add some sections to your porch. Earlier you lot become to the hardware store to buy lumber, you demand to decide the unit composition (the material between two big uprights). Y'all count how many posts, how many boards, how many runway—then you decide how many sections you want to add together—before yous summate the corporeality of building fabric needed for your porch expansion.
Mole Ratios
Stoichiometry problems tin be characterized past two things: (1) the information given in the problem, and (2) the data that is to exist solved for, referred to as the unknown. The given and the unknown may both be reactants, both be products, or one may be a reactant while the other is a product. The amounts of the substances tin can exist expressed in moles. However, in a laboratory state of affairs, it is common to determine the amount of a substance by finding its mass in grams. The corporeality of a gaseous substance may be expressed by its volume. In this concept, we will focus on the type of problem where both the given and the unknown quantities are expressed in moles.

Chemic equations express the amounts of reactants and products in a reaction. The coefficients of a balanced equation can represent either the number of molecules or the number of moles of each substance. The production of ammonia \(\left( \ce{NH_3} \correct)\) from nitrogen and hydrogen gases is an important industrial reaction chosen the Haber process, later on German pharmacist Fritz Haber.
\[\ce{N_2} \left( k \right) + iii \ce{H_2} \left( yard \correct) \rightarrow 2 \ce{NH_3} \left( yard \correct)\]
The balanced equation can exist analyzed in several ways, as shown in the figure below.
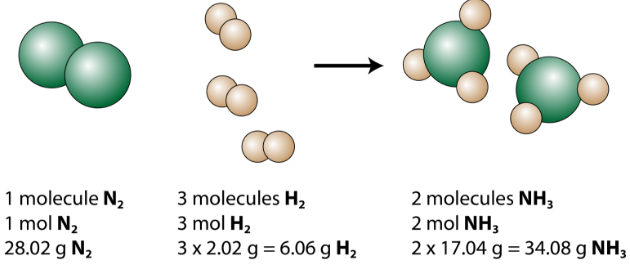
We meet that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to grade two molecules of ammonia. This is the smallest possible relative amount of the reactants and products. To consider larger relative amounts, each coefficient can be multiplied by the same number. For example, x molecules of nitrogen would react with xxx molecules of hydrogen to produce 20 molecules of ammonia.
The almost useful quantity for counting particles is the mole. So if each coefficient is multiplied by a mole, the balanced chemical equation tells united states of america that ane mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia. This is the conventional way to interpret any balanced chemical equation.
Finally, if each mole quantity is converted to grams past using the molar mass, we tin see that the police force of conservation of mass is followed. \(1 \: \ce{mol}\) of nitrogen has a mass of \(28.02 \: \text{g}\), while \(iii \: \text{mol}\) of hydrogen has a mass of \(6.06 \: \text{g}\), and \(2 \: \text{mol}\) of ammonia has a mass of \(34.08 \: \text{one thousand}\).
\[28.02 \: \text{grand} \: \ce{N_2} + 6.06 \: \text{g} \: \ce{H_2} \rightarrow 34.08 \: \text{m} \: \ce{NH_3}\]
Mass and the number of atoms must be conserved in any chemic reaction. The number of molecules is not necessarily conserved.

A mole ratio is a conversion factor that relates the amounts in moles of any 2 substances in a chemical reaction. The numbers in a conversion gene come from the coefficients of the balanced chemic equation. The following six mole ratios can be written for the ammonia forming reaction to a higher place.
\[\brainstorm{array}{ccc} \dfrac{one \: \text{mol} \: \ce{N_2}}{3 \: \text{mol} \: \ce{H_2}} & or & \dfrac{3 \: \text{mol} \: \ce{H_2}}{ane \: \text{mol} \: \ce{N_2}} \\ \dfrac{one \: \text{mol} \: \ce{N_2}}{2 \: \text{mol} \: \ce{NH_3}} & or & \dfrac{2 \: \text{mol} \: \ce{NH_3}}{1 \: \text{mol} \: \ce{N_2}} \\ \dfrac{three \: \text{mol} \: \ce{H_2}}{2 \: \text{mol} \: \ce{NH_3}} & or & \dfrac{2 \: \text{mol} \: \ce{NH_3}}{three \: \text{mol} \: \ce{H_2}} \cease{array}\]
In a mole ratio problem, the given substance, expressed in moles, is written starting time. The appropriate conversion factor is called in lodge to convert from moles of the given substance to moles of the unknown.
Instance \(\PageIndex{1}\)
How many moles of ammonia are produced if 4.twenty moles of hydrogen are reacted with an backlog of nitrogen?
Solution
Step 1: Listing the known quantities and programme the problem.
Known
Unknown
The conversion is from \(\text{mol} \: \ce{H_2}\) to \(\text{mol} \: \ce{NH_3}\). The problem states that there is an excess of nitrogen, so we do not need to be concerned with any mole ratio involving \(\ce{N_2}\). Cull the conversion factor that has the \(\ce{NH_3}\) in the numerator and the \(\ce{H_2}\) in the denominator.
Step ii: Solve.
\[4.twenty \: \text{mol} \: \ce{H_2} \times \dfrac{2 \: \text{mol} \: \ce{NH_3}}{3 \: \text{mol} \: \ce{H_2}} = two.eighty \: \text{mol} \: \ce{NH_3}\]
The reaction of \(four.20 \: \text{mol}\) of hydrogen with backlog nitrogen produces \(two.eighty \: \text{mol}\) of ammonia.
Step 3: Think near your result.
The result corresponds to the 3:2 ratio of hydrogen to ammonia from the balanced equation.
Summary
- Mole ratios allow comparing of the amounts of any 2 materials in a counterbalanced equation.
- Calculations can be made to predict how much production can exist obtained from a given number of moles of reactant.
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/12%3A_Stoichiometry/12.02%3A_Mole_Ratios
0 Response to "what ratio is used to carry out each conversion"
Postar um comentário